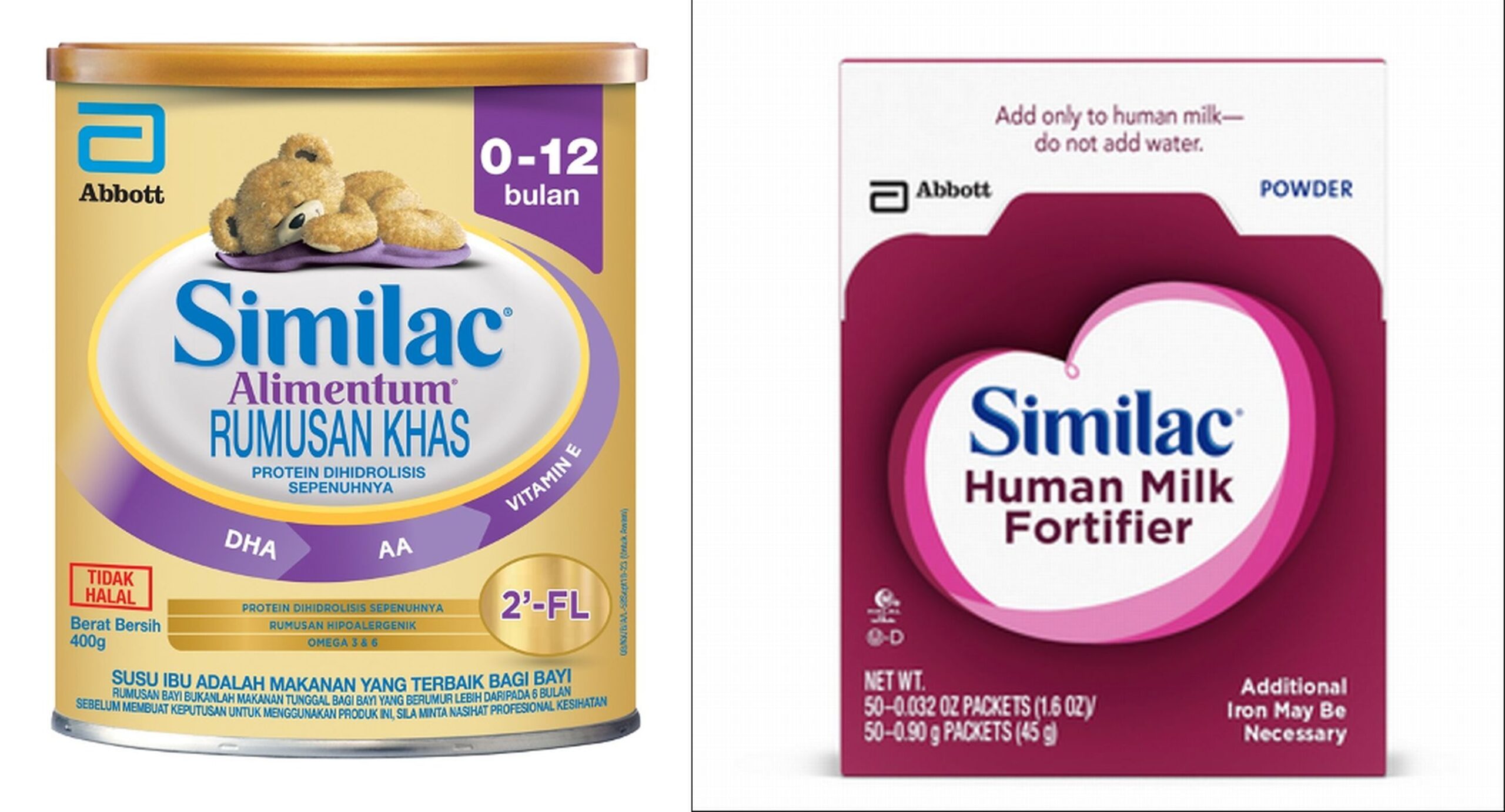
KUALA LUMPUR, Feb 25 – The Ministry of Health (MOH) has announced the recall of two infant formula products, which were discovered to be contaminated with Cronobacter sakazakii or Salmonella Newport.
The products, Alimentum (400g) and Human Milk Fortifier (0.9g x 50 sachets) by Abbott, were manufactured in a facility in Sturgis, United States.
Cronobacter bacteria can cause severe, life-threatening infections (sepsis) or meningitis (an inflammation of the membranes that protect the brain and spine), according to the US Food and Drug Administration (FDA).
Symptoms of sepsis and meningitis may include poor feeding, irritability, temperature changes, jaundice, shortness of breath, and abnormal movements. Cronobacter infection may also cause bowel damage and may spread through the blood to other parts of the body.
Salmonella can cause gastrointestinal illness and a fever called salmonellosis. Most people with salmonellosis develop diarrhoea, fever and abdominal cramps.
More severe cases of salmonellosis may include a high fever, aches, headaches, lethargy, a rash, blood in the urine or stool, and in some cases, may become fatal, said the FDA in a media release.
Products involved in the recall have batch numbers beginning with digits 22 to 37 on the barcode in containers numbered K8, SH, or Z2, and have an expiration date of April 1, 2022 onwards.
MOH has also imposed an auto-rejection of the product at all entrances to the country. All distributors of the products including online retailers who still have stock of the infant formulas must immediately cease sales and contact the nearest district health office.
The action taken by MOH is in response to the FDA’s investigation following consumer complaints of Cronobacter sakazakii and Salmonella Newport infections.
In the US, Abbott voluntarily recalled its infant formula products, Similac, Alimentum and EleCare, following four complaints from three states related to Cronobacter sakazakii in infants who had consumed them.
The company said it found evidence of Cronobacter in non-product contact areas at its Sturgis, Michigan facility.
All four cases related to these complaints were hospitalised, and Cronobacter may have contributed to a death in one case, according to the FDA. It has initiated an on-site inspection at the facility.